Liberate Medical Reports Positive Pilot Trial Results of VentFree™ Respiratory Muscle Stimulator
Second trial to demonstrate improved outcomes in mechanically ventilated patients
CRESTWOOD, KY, 16th November 2020 – Applying non-invasive electrical stimulation to the expiratory abdominal muscles may reduce abdominal muscle atrophy and markedly reduce the number of days adult patients require mechanical ventilation – a potentially important finding for weaning ventilated patients, including seriously ill COVID-19 patients, and improving patient outcomes.
Liberate Medical today announced the results of a second randomized controlled pilot trial of its proprietary medical device, the VentFree Respiratory Muscle Stimulator, the only non-invasive and only breath-synchronized neuromuscular electrical stimulation device used to address respiratory muscle atrophy in mechanically ventilated patients. The trial was published in the journal Critical Care.
“VentFree is a promising and unique technique for providing respiratory muscle protective ventilation,” commented first author Annemijn Jonkman, MSc, Amsterdam UMC.
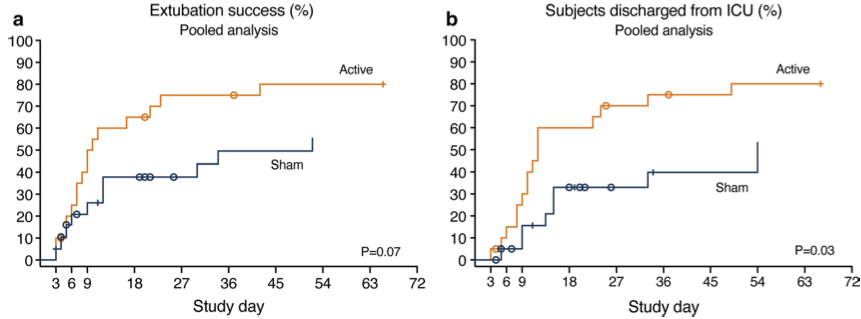
The trial, conducted in the Netherlands, was assessor blinded, randomized, sham-controlled and included twenty participants. The results showed that the intervention was safe and feasible with a total treatment compliance rate of 91.1%. The results from this EU study were combined with the results of a similar trial in Australia that was publishedlast year. In the pooled analysis:
Median ventilation duration and ICU length of stay in the total study population were 10 versus 52 (P = 0.07), and 12 versus 54 (P = 0.03) days for the intervention versus sham group.
Total abdominal expiratory muscle thickness was increased at day 3 in the intervention versus sham group (P = 0.02).
Sixteen (16/20) patients were successfully extubated in the intervention group vs. ten patients (10/20) in the sham group;
During ICU stay, 3/20 patients died in the intervention group versus 8/20 in the sham group (P = 0.16)
Prof. Leo Heunks, MD, PhD, Amsterdam UMC, coordinating investigator for the study commented, “We’re very encouraged by these results, which indicate that the VentFree has the potential to meaningfully improve patient outcomes both during the COVID-19 pandemic and beyond.”
“We’re excited to announce these results” said Angus McLachlan, Liberate Medical Chief Executive Officer. “Reducing the time patients are on mechanical ventilation may reduce the risks of prolonged mechanical ventilation, which include muscle weakness, hospital-acquired infections, and deteriorated quality of life – a frequent outcome for so-called COVID-19 ‘long-haulers.’”
VentFree recently received FDA Emergency Use Authorization for use during the COVID-19 pandemic. Last year, VentFree received FDA Breakthrough Device Designation and CE marking in the European Union.
About Liberate Medical
Liberate Medical is a medical device company that develops neuromuscular electrical stimulation technology to improve the quality and reduce the cost of care for patients with pulmonary disorders. For more information please visit https://liberatemedical.com.
About the VentFree Respiratory Muscle Stimulator
Invasive mechanical ventilation commonly weakens the breathing muscles, increasing the need for further ventilator support. The VentFree respiratory muscle stimulator contracts the abdominal muscles in mechanically ventilated patients using proprietary non-invasive electrical stimulation applied in synchrony with exhalation. As electrical stimulation does not require patient participation, therapy can begin from day one of mechanical ventilation while patients are sedated or delirious. This breakthrough therapy, which is quick and simple to use, is intended to reduce abdominal muscle atrophy and the time taken to liberate patients from mechanical ventilation.
Reduced days on mechanical ventilation can potentially lead to reduced morbidity and mortality, improved quality of life and considerable savings for the health care provider.
Media Contact
Morgan McKenna
(617) 482-0042